In this section
Learn:
- Why certified health IT is important to your practice
- How to compare certified health IT products
- How application program interfaces (APIs) can ease information exchange
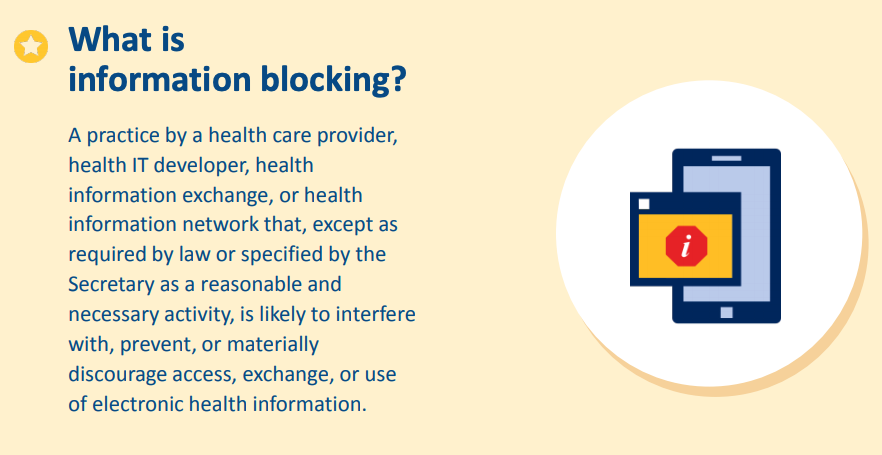
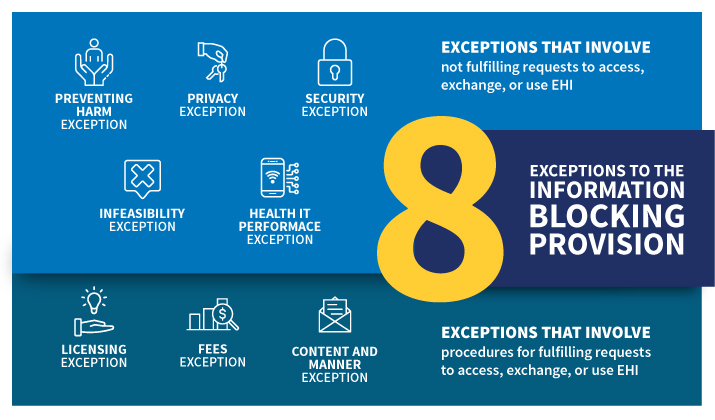
- How to recognize information blocking
- How to report issues with certified health IT products to the Office of the Assistant Secretary for Technology Policy (ASTP)
How can certified health IT help my practice?
Certified health IT can help your practice by:
- Making electronic prescribing available — which is safer, cheaper, and more convenient for clinicians and patients
- Supporting standardized application programming interface (API) technology, electronic transitions of care, closing referral loops, and giving clinicians straightforward and secure access to their patients' records from outside organizations
- Making the process for patients to get their personal health information less time-consuming and tedious for all parties while maintaining confidentiality
- Automating the process of sending data to immunization registries
- Facilitating the reporting of electronic clinical quality measures to the Centers for Medicare & Medicaid Services (CMS)
History of the ONC Health IT Certification Program
In 2009, the Health Information Technology for Economic and Clinical Health (HITECH) Act was signed into law as part of the American Recovery and Reinvestment Act (ARRA). The HITECH Act established the Office of the National Coordinator for Health Information Technology (ONC) — now the Office of the Assistant Secretary for Technology Policy and the Office of the National Coordinator for Health IT (ASTP) — as the principal federal entity charged with coordinating nationwide efforts to implement and use the most advanced health IT and electronic exchange of health information.
ASTP oversees the Health IT Certification Program for Health IT Modules. The Certification Program sets several nationwide requirements including:
- Health IT standards
- Implementation specifications
- Certification criteria
The HITECH Act also established the Electronic Health Record (EHR) Incentive programs, or Meaningful Use programs (now known as the Promoting Interoperability Programs). In the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), Congress made meaningful use of certified electronic health record technology (CEHRT) (now known as Promoting Interoperability) part of the Merit-based Incentive Payment System (MIPS) for eligible clinicians.
Administered by CMS, the incentive programs encourage eligible professionals, hospitals, and critical access hospitals to adopt, implement, and use CEHRT.
To participate in the CMS incentive programs, clinicians must demonstrate their “meaningful use” of CEHRT. That means meeting certain requirements — such as proving that you capture and share patient data using CEHRT, have completed a security risk analysis, and have not knowingly and willfully limited or restricted the compatibility or interoperability of your CEHRT.
ASTP and CMS established these requirements so that clinicians can electronically send and receive patient care information in a consistent, usable manner. Other programs that call for using CEHRT include MIPS and Advanced Alternative Payment Models (APMs).
ASTP has released several final rules that support the certification and availability of certified health IT for its encouraged and required use under other federal, state, and private programs. CMS has released similar changes to their programs that align with ASTP's updates. Each of these updates has enhanced the Certification Program framework by continuing to support clinicians in their efforts to:
- Increase care coordination, engage with patients, and improve outcomes
- Advance the development and use of health IT capabilities and establish expectations for data sharing
- Advance equity, innovation, and interoperability
- Support access to — and the exchange and use of — electronic health information (EHI)
Improving clinician access to certified technology will help make patient data consistently available to the right people at the right time and place.
For clinicians, implementers, and developers seeking more information about the Certification Program structure, key players, operations, and history, please review the following resources:
Why is the use of certified health IT important to your practice?
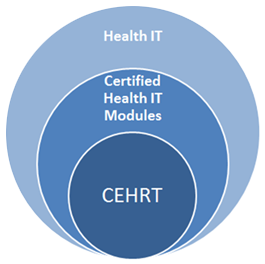
Certified health IT plays a vital role in establishing a nationwide, connected, and interoperable health information infrastructure. Health IT Modules certified under the Certification Program are listed on the Certified Health IT Product List (CHPL).
Using certified health IT — including standards-based APIs, electronic exchange of clinical care documents, and other standards-based transactions such as e-prescribing — improves care coordination. Certification provides a baseline assurance that a Health IT Module will perform clinical care and data exchange functions in accordance with interoperability standards and user-centered design. The benefits of standard data capture and interoperable exchange of information include enhanced patient safety, usability, privacy, and security.
Standards incorporated into the Certification Program include vocabulary code sets, like SNOMED-CT®, which ensure consistent clinical terminology between systems. Standards for structuring clinical content include the Consolidated Clinical Document Architecture (C-CDA), which is discussed in Section 1 of this playbook. The C-CDA allows different health IT systems to send and receive a patient's clinical care summary while retaining the same meaning across systems. Other standards for exchanging patients' health information include the Fast Healthcare Interoperability Resources (FHIR®) standard, which enables the APIs that allow health IT systems to access and share certain data elements — such as medications, allergies, or lab results — in a flexible, real-time, and standardized way.
Certain health care payment programs — including the Promoting Interoperability Programs for hospitals and MIPS under the Quality Payment Program for eligible clinicians (formerly the EHR Incentive or Meaningful Use programs) — require the use of certified health IT. CMS defines CEHRT as health IT that meets the minimum set of required certification functionalities needed for program participants to comply with incentive program requirements.
What certified health IT will I need to participate in certain CMS programs?
Certain criteria are leveraged to meet CMS measures and outcomes outlined in their incentive programs. CEHRT defines key criteria to which a Health IT Module should be certified to meet incentive program requirements. To learn more about what certified health IT you will need to participate in certain CMS programs, please visit the CMS page on value-based programs to find information about specifications that may apply to you.